BY: JENNIFER CORSO, BA, MA, Darwin Brands
The cannabis plant is comprised of much more than cannabinoids like THC and CBD. It is rich in many phytochemicals, including terpenes and terpenoids. These volatile, odorous organic compounds act as antioxidant, anti-fungal and anti-bacterial agents, protecting plants against disease and infection, and are shining in the current spotlight for their therapeutic value.
But What Exactly Are Terpenes?
Phytochemicals are a group of biologically-active compounds found in plants that provide some type of health benefit. They are not required by the human body to sustain life, however, many of them have proven to aid in the prevention of disease and reduce the risk of developing cancer. Terpenes, along with polyphenols, antioxidants and flavonoids are all types of phytochemicals, and they are just a few of many classifications that are currently accounted for.
Terpenes are among several groups of low molecular weight chemicals responsible for the aromas associated with flowers, citrus, and conifers – they can be attributed to why lilies and lilacs smell distinctly sweet and floral [(E)-?-ocimene], and why that nug of Lemon Train Haze has a delicious, citrus-y zing to it (D- and L-limonene). The variety of odors attract pollinators and fight off predators, assisting plants in both reproduction and survival.
Terpenes and terpenoids are classified as lipids, similar in structure to steroids, squalene and cannabinoids. They are biologically synthesized from isopentenyl pyrophosphate (IPP), a molecular intermediate created in the HMG-CoA reductase pathway (the same pathway that produces cholesterol). Every terpene contains a multiple of five carbons and eight hydrogen atoms (this is called the C₅ Rule or Isoprene Rule, discovered in 1953). Variations in structure include oxygen atoms or cycling rings.
The Science of Terpenes
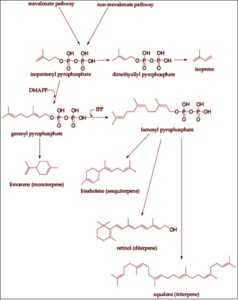
Terpenes are classified based on the number of carbon atoms they are comprised of. Monoterpenes contain 10 carbon atoms, are volatile and fragrant, and the chief constituents of essential oils. Sesquiterpenes are the most diverse group of terpenes, each containing 15 carbons and found is essential oils and resins. As the number of isoprene units increases, diterpenes, sesterterpenes, triterpenes, carotenoids are formed, and eventually rubber (>100 isoprene units!)
There is substantial scientific evidence for the suggestion that terpenes aid in disease prevention, although there many mechanisms we have yet to understand. Because terpenes are found in anything from eucalyptus to blood oranges, they are easier to study, legally and independently from cannabis.
It wasn’t until 1998 that researchers began investigating the therapeutic potential of terpenes in cannabis and applying them toward what we refer to as the “entourage effect.” This is the synergistic interplay of phytochemicals within the plant that may elicit a response different or greater than individual compounds themselves. The effect is seen most clearly between cannabinoids CBD and THC, and is most supported by researchers such as Dr. Ethan Russo, who published a notable article on the idea in the British Journal of Pharmacology in 2011.
Curious about learning more? Here is a spotlight on three interesting terpenes found in cannabis, with supporting scientific evidence on their therapeutic value.
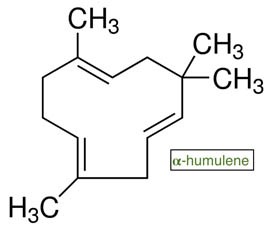
ALPHA HUMULENE
Alpha-humulene was first discovered in the lupulin glands (trichomes) of the flower or cone of the female hop plant, Humulus lupulus, playing a notable role in the plant’s characteristic smell. Cannabis’ sister-from-another-mister, hops are bred to elicit a plethora of different aromatic and flavor profiles. Because of the antiseptic ability of the ?-acids (humulones), hops were historically incorporated in beer as a preservative, but they also balance the sweetness of the malt sugars in cooked wort. The more aromatic varieties, like noble hops, are lower in ?-acids and higher in aromatics like ?-humulene – up to 40%, delivering a sensory experience familiar to both beer and cannabis connoisseurs alike.
- Classification and chemistry: A monocyclic sesquiterpene and farnesyl diphosphate (FPP) derivative, with the molecular formula C₁₅H₂₄. Also known as ?-caryophyllene, an opened-ring isomer of ?-caryophyllene. A primary terpene in cannabis essential for growth and development.
- Aroma and flavor: Robust, woody, spicy; also found in common sage, ginger and ginseng.
- Cannabis strains high in ?-humulene: White Widow, Original Glue, GSC
- Main effects:
○ Anti-inflammatory, illustrated through the reduction of eosinophil migration and inhibition of IL-5, NF?B and AP-1 in the lung during aerosol application; similar to dexamethasone utilization.
○ Antineoplastic by inducing cytotoxicity through increased ROS production and decreased cellular glutathione concentration. May also work synergistically with the chemotherapeutic agent doxorubicin in certain ovarian INcancer cell lines.
○ Antiseptic (antibacterial, antifungal)
- Entourage effect: May work synergistically with trans-caryophyllene as an anti-inflammatory and anti-cancer agent. May work with tetrahydrocannabivarin (THCV) to suppress appetite.
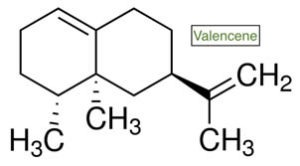
VALENCENE
Valencene – you guessed it – is primarily found in Valencia oranges, and is the precursor to the notable odorant found in grapefruit, nootkatone. Looking to combat the skin-deep signs of aging? An interesting study published in 2016 illustrated the effects of valencene on UVB radiation. The researchers found that valencene interacts with TRPV1 and ORAI1 calcium channels, assisting in the regulation of photoaging and prevention of skin pigment changes and inflammation. There is some say that valencene may also assist in promoting a calm mood, but more research needs to be done in this area in particular.
- Classification and chemistry: A bicyclic sesquiterpene, (+)-valencene has the molecular formula C₁₅H₂₄. It is used widely to synthesize nootkatone, and may be biotransformed by various microorganisms through oxidation, as well as CYP-450 found in the liver.
- Aroma and flavor: Sweet citrus, oranges, grapefruit
- Cannabis strains containing (+)-valencene: ACDC, Agent Orange, Tangie
- Main effects:
○ Antiproliferative: In two lines of ovarian cancer, and two lines of lymphoblast cancer, valencene was shown to successfully enhance the effects of chemotherapeutic agent doxorubicin by increasing the accumulation of the drug in the treated cells. It’s also been shown to improve efficacy in colon cancer treatment.
○ Antioxidant activity, highest as a constituent of sweet orange oil, however successful as an isolated compound. May have cytotoxic effects.
- Entourage effect: May work best as an antioxidant with terpenes such as linalool and octanal.
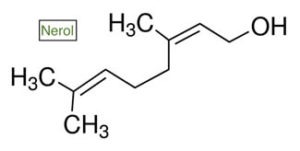
NEROL
Nerol was originally discovered as a constituent of the prized essential oil neroli, which is extracted and steam distilled from the delicate and sweet blossoms of the bitter Seville orange tree. The molecular structure is close to muguet – change the location of one methyl group and the aroma changes from sweet citrus to lily of the valley. It is known for its stress-relieving properties, and has been shown to reduce anxiety in mice through decreases in motor activity, similar to the effects of diazepam. Found in small amount in cannabis, nerol has been shown to be an effective insect repellent, assisting in plant defenses. And if you’re a fan of wine, nerol, along with its stereoisomer geraniol, is an important part of the flavor nuances in young Muscat varieties.
- Classification and chemistry: A linear, volatile monoterpene and alcohol with the molecular formula C₁₀H₁₈O. Nerol is the cis (2Z) stereoisomer of geraniol. It can be synthesized from pyrolysis of ?-pinene, and from ?-myrcene.
- Aroma and flavor: fruity, sweet rose, citrus, raspberry, deep; also found in lemongrass, rose, orange blossoms, lavender.
- Cannabis strains containing nerol: There aren’t any strains that feature nerol in high concentrations, however may be present in strains containing geraniol, such as Lavender.
- Main effects:
○ Anxiolytic – the mechanism may be similar to the CNS-attenuating effects of benzodiazepines on GABA channels.
○ Antibacterial against several strains of Gram-(+) and Gram-(-) bacteria, to include Escherichia coli, Mycobacterium tuberculosis, and Salmonella typhimurium, among others. More info, here.
- Entourage effect: May work synergistically with linalool and geraniol to induce a relaxing/sedating effect, and also ameliorate the onset of anxiety associated with THC.
Until next time… smell ya later!
Sources:
https://truphys.com/antioxidants-and-training/
Egbuta, M., McIntosh, S., Waters, D., Vancov, T., & Liu, L. (2017). Biological importance of cotton by-products relative to chemical constituents of the cotton plant. Molecules, 22(1), 93.
https://www.scientificamerican.com/article/why-do-flowers-have-scent/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2785529/pdf/bph0158-1074.pdf
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2003-39695
https://www.ncbi.nlm.nih.gov/pubmed/28632185
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3684331/pdf/i1524-5012-13-2-214.pdf
https://www.sciencedirect.com/science/article/pii/S0254629910001559#bbib95
https://www.tandfonline.com/doi/abs/10.1080/10412905.2007.9699283
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1750-3841.2012.02924.x
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6152637/pdf/molecules-22-01021.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946/

— A US Army veteran, Jenny Corso is the Lead Scientist for Darwin Brands. She holds a Masters’ degree in Kinesiology (Exercise Physiology) with a secondary concentration in Biochemistry. She taught at both California State University Chico and Arizona State University, and presented her research at conferences for American Heart Association Scientific Sessions, the US Cannabis Conference 2018 and the American College of Sports Medicine. She also presents science lectures for Women Grow Phoenix.


